

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Bos taurus) | BDBM36008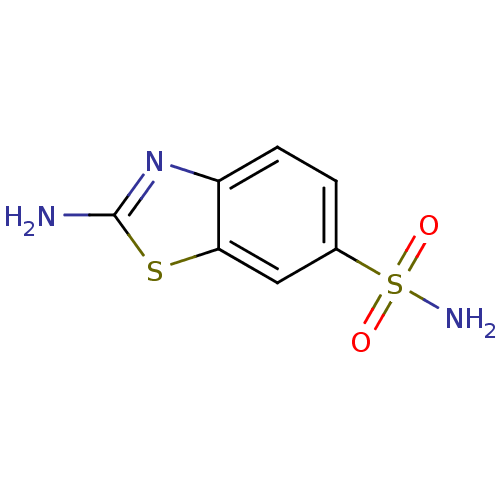 (2-amino-1,3-benzothiazole-6-sulfonamide | SPR_12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | 383 | n/a | 5.10E+4 | 0.0196 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36005 (2,3-dihydro-1H-indole-5-sulfonamide | SPR_9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | n/a | n/a | n/a | 3.11E+3 | n/a | 1.88E+4 | 0.0585 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36007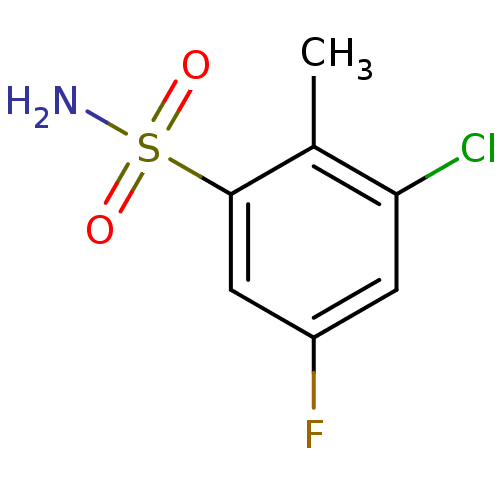 (3-chloro-5-fluoro-2-methylbenzene-1-sulfonamide | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | n/a | n/a | n/a | 96.8 | n/a | 7.88E+5 | 0.0763 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36006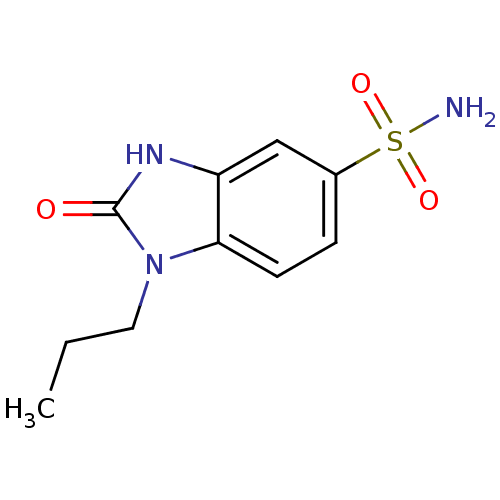 (2-oxo-1-propyl-2,3-dihydro-1H-1,3-benzodiazole-5-s...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | n/a | 197 | n/a | 4.75E+5 | 0.0935 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36004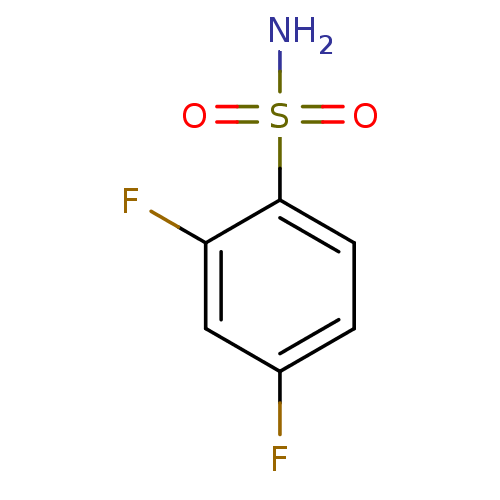 (2,4-difluorobenzene-1-sulfonamide | SPR_8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | 394 | n/a | 2.72E+5 | 0.107 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36002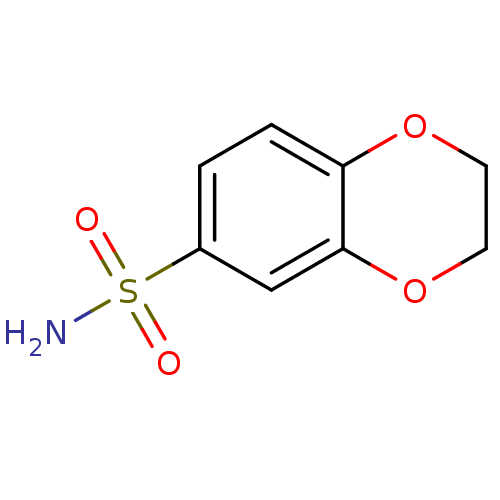 (2,3-dihydro-1,4-benzodioxine-6-sulfonamide | SPR_6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | n/a | n/a | n/a | 817 | n/a | 1.34E+5 | 0.109 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36003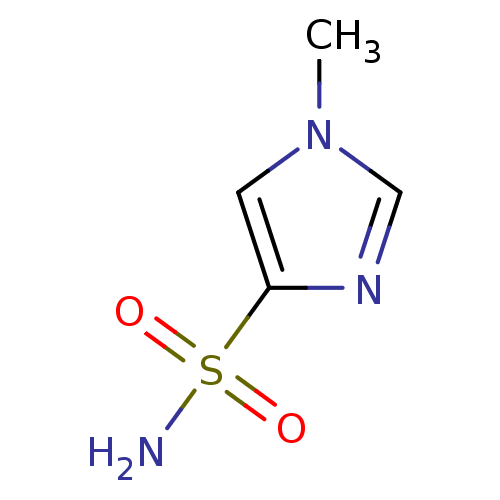 (1-methyl-1H-imidazole-4-sulfonamide | SPR_7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | n/a | n/a | n/a | 3.53E+4 | n/a | 4.65E+3 | 0.164 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36001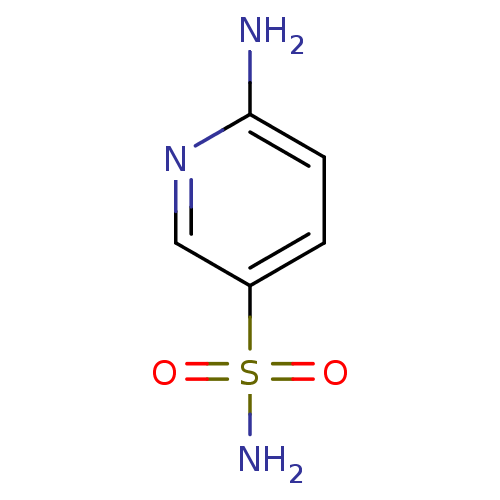 (6-aminopyridine-3-sulfonamide | SPR_5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | n/a | n/a | n/a | 1.08E+4 | n/a | 1.88E+4 | 0.203 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM35999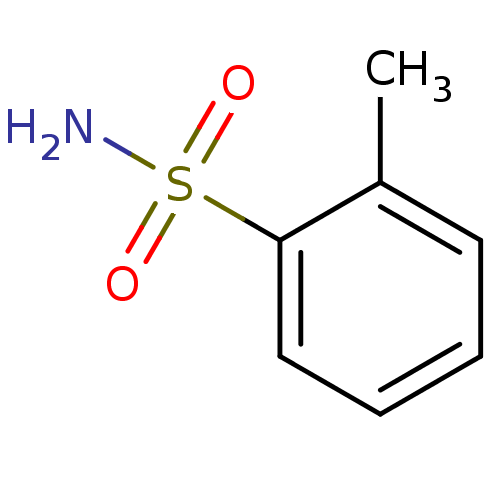 (2-methylbenzene-1-sulfonamide | SPR_3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | n/a | n/a | n/a | 2.18E+3 | n/a | 1.10E+5 | 0.240 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM36000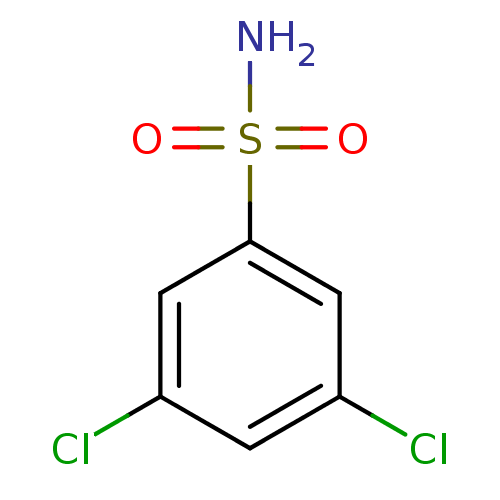 (3,5-dichlorobenzene-1-sulfonamide | SPR_4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | n/a | 287 | n/a | 9.02E+5 | 0.259 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM10888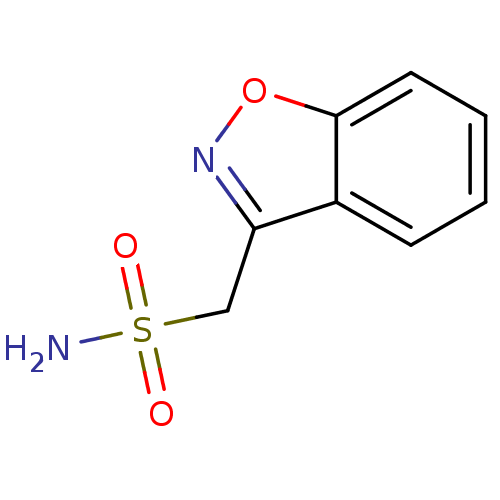 (1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | n/a | 4.26E+3 | n/a | 1.43E+5 | 0.611 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM35997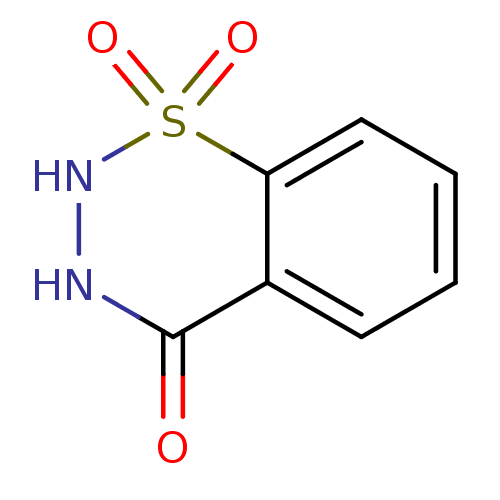 (1,4-dioxo-1,2,3,4-tetrahydro-1,2,3-benzothiadiazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | n/a | n/a | n/a | 3.89E+4 | n/a | 4.99E+4 | 1.94 | 7.4 | 25 | |
University of Dundee | Assay Description SPR biosensor assay Biacore T100 | ACS Med Chem Lett 1: 44-8 (2010) BindingDB Entry DOI: 10.7270/Q2BC3WXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163864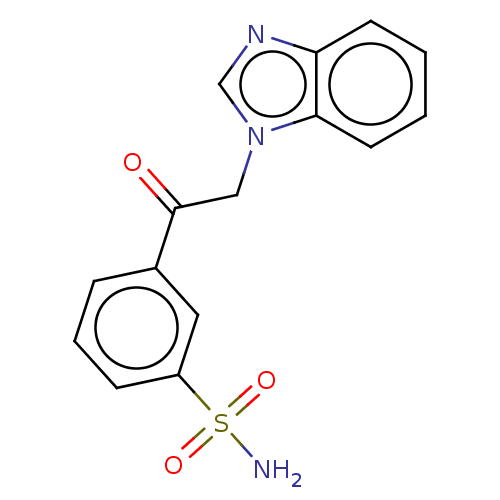 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380144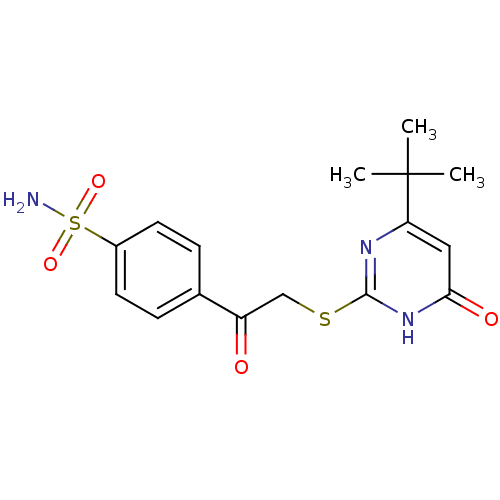 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 5.70E+4 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163864 (3-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 7.20E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50329822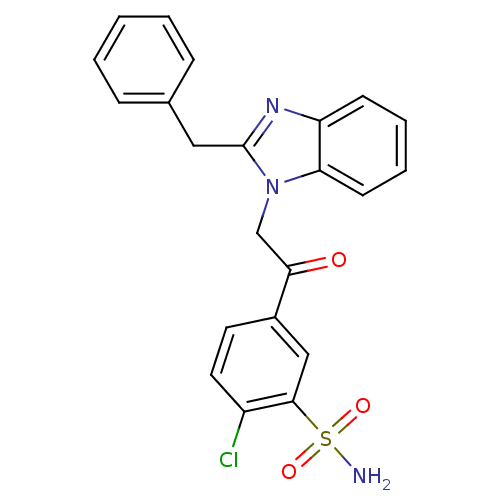 (5-[(2-Benzyl-1H-benzimidazol-1-yl)acetyl]-2-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.00E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163861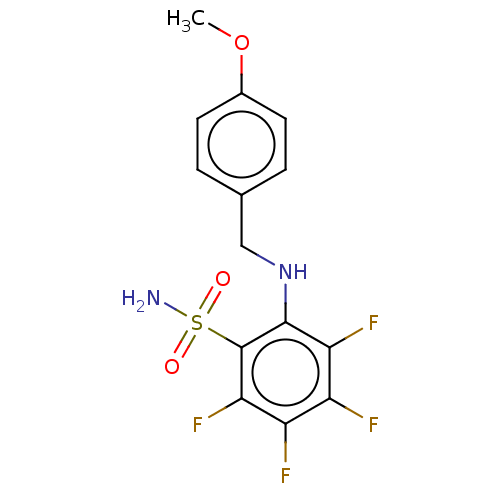 (CHEMBL3799945 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.10E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50380140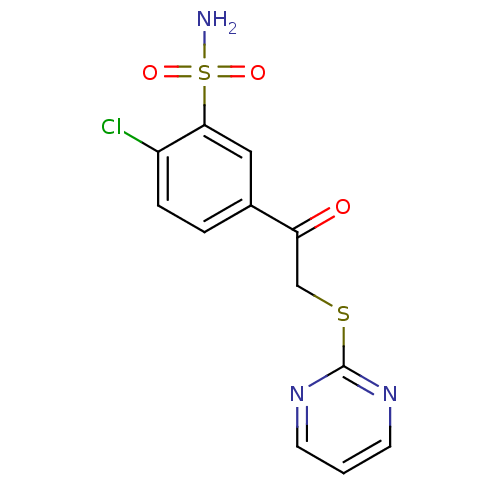 (CHEMBL2011155 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.20E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163859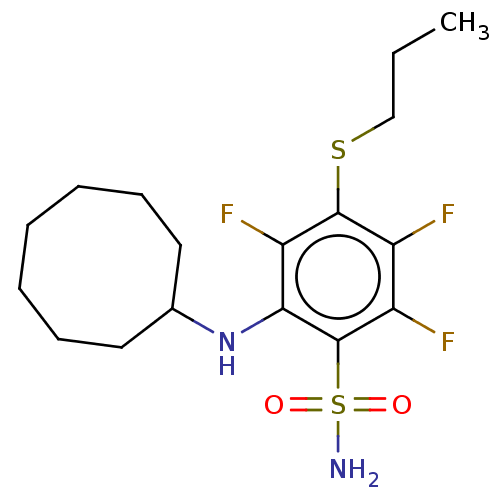 (CHEMBL3797835 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50329832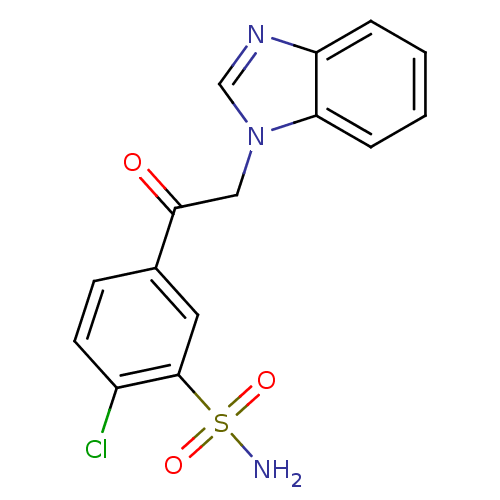 (5-(1H-Benzimidazol-1-ylacetyl)-2-chlorobenzenesulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163866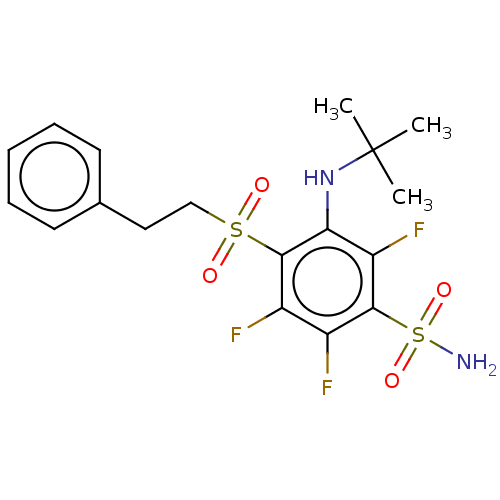 (CHEMBL3798473 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163867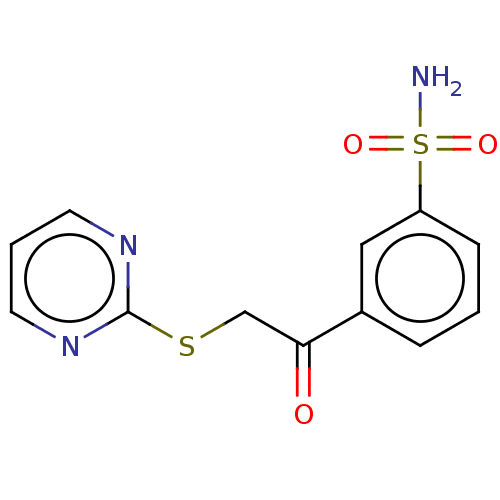 (CHEMBL2443189 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50380148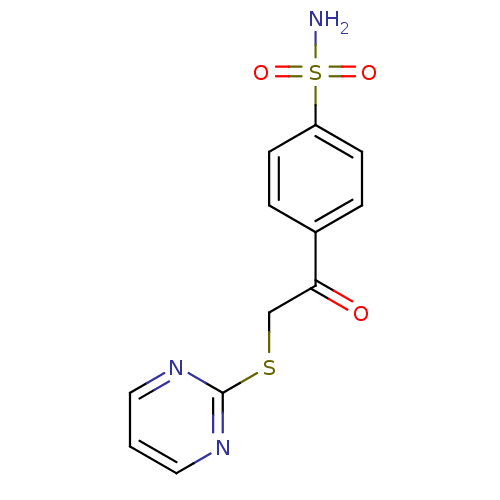 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163862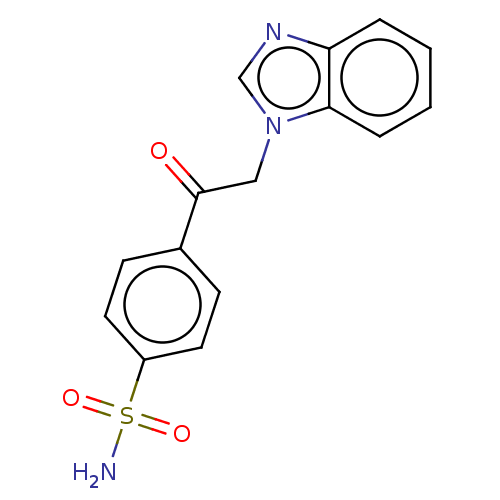 (4-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.50E+5 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163862 (4-(1H-benzimidazol-1-ylacetyl)benzenesulfonamide (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163858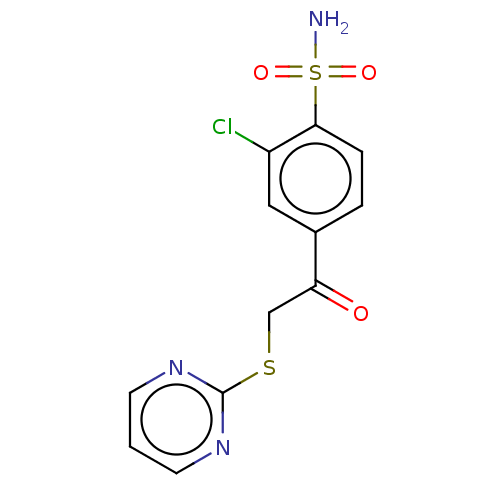 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.10E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163863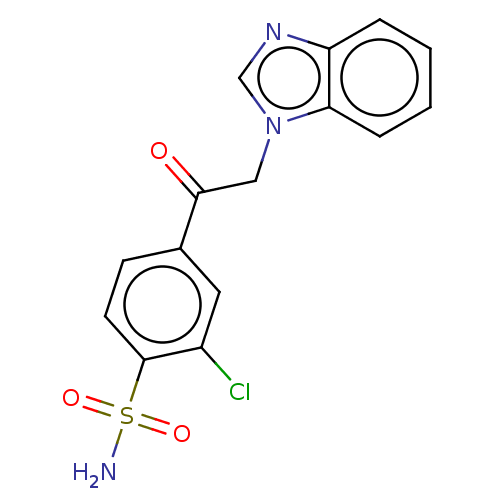 (4-(1H-benzimidazol-1-ylacetyl)-2-chlorobenzenesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.30E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description The GSK3beta primary screen was conducted in assay ready 1536 plates (Aurora 29847) that contain 2.5 mL/well of 10 mM compound. Human GSK3beta as a G... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.60E+5 | 6.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 6 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163863 (4-(1H-benzimidazol-1-ylacetyl)-2-chlorobenzenesulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50163865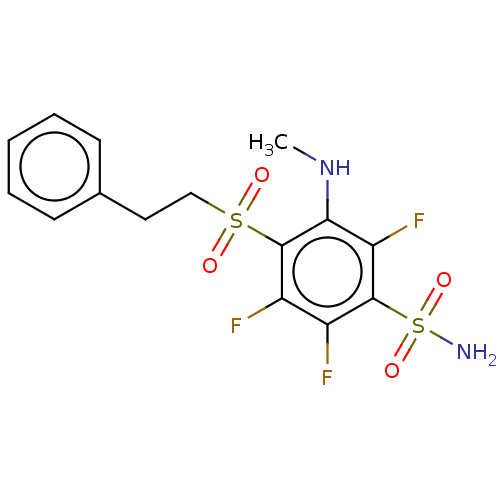 (CHEMBL3799577 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 3.90E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 4.00E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 6.30E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA1 expressed in Escherichia coli assessed as association rate constant after 30 secs by surface plasmon resona... | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380140 (CHEMBL2011155 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 8.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163860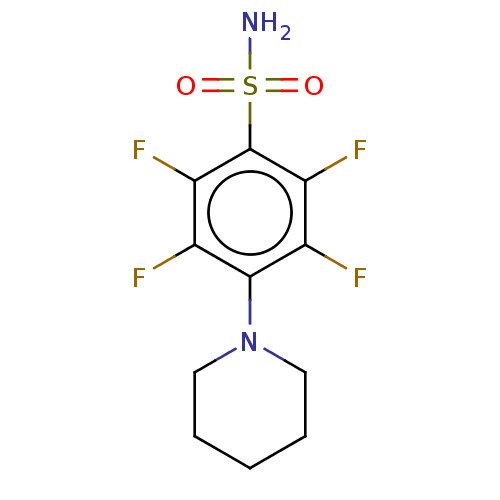 (CHEMBL3797893 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 8.80E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380144 (CHEMBL2010994 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.10E+6 | 8.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 8 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50429922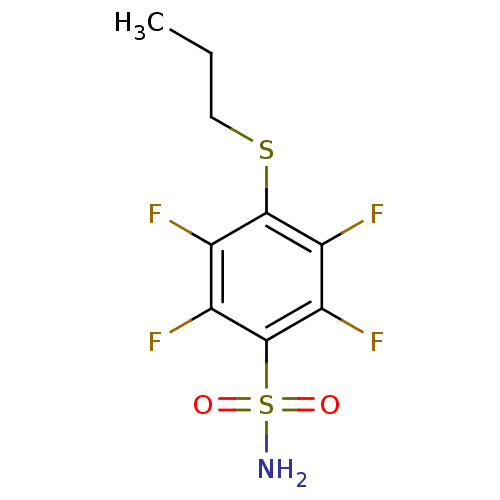 (CHEMBL2333418 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.30E+6 | 8.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 8 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163865 (CHEMBL3799577 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.50E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.60E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50163858 (CHEMBL2443188 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 1.80E+6 | 8.0 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant at pH 8 by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50380148 (CHEMBL2011156 | carbonic anhydrase (CA) inhibitors...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | 2.00E+7 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to human recombinant CA12 catalytic domain assessed as association rate constant after 30 secs by surface plasmon resonance assay | J Med Chem 59: 2083-93 (2016) Article DOI: 10.1021/acs.jmedchem.5b01723 BindingDB Entry DOI: 10.7270/Q2571DWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||